1.5 Introducing the Fission Process
Now that we know how to compute binding energy, letís answer the question we asked on the previous page. If you recall, we wondered what would happen if a large atom was split into two smaller atoms. The process by which we do this is called fission.
Fission occurs when a large atom absorbs an extra neutron. When this happens the
nucleus becomes unstable and the atom splits into two smaller parts. Weíll cover why this happens in a
moment, but for right now, letís just accept that it happens. The two elements which emerge from a fission
event are called fission products. These products vary according to a probability curve, but right now thatís not
important. We are going to look at a specific event.For the purposes of this discussion, weíre going to imagine a
![]() atom absorbs a neutron and splits into
atom absorbs a neutron and splits into
![]() and
and ![]() and three neutrons.
and three neutrons.
This is the nuclear equation that represents that event:
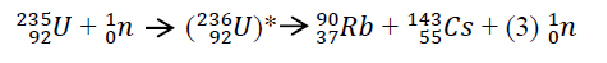
The asterisks after the signifies that this nucleus is in an excited state and unstable. This excited state will lead to the splitting of the Uranium atom into a Rubidium atom and Cesium atom. So letís analyze this equation from a mass defect standpoint. We will first examine the mass on the far left side and compare it to the mass on the far right hand side.
The mass on the far left hand side is equal to the mass of a ![]() atom plus the mass of a neutron.
atom plus the mass of a neutron.
We know from our previous example that the mass of ![]() is 235.0440 AMU.
is 235.0440 AMU.
We also know that the mass of a neutron is 1.00866 AMU.
Therefore: 235.0440 + 1.00866 = 236.0527 AMU. (Mass of Left Side of Equation)
The mass on the far right hand side is equal to the mass of a ![]() atom plus the mass of a
atom plus the mass of a ![]() atom plus three neutrons.
atom plus three neutrons.
By consulting a table, we know that the mass of ![]() is 89.915 AMU.
is 89.915 AMU.
By consulting a table we also know that the mass of ![]() is 142.927 AMU.
is 142.927 AMU.
Therefore: 89.915 + 142.927 + (3*1.00866 ) = 235.868AMU (Mass of Right Side of Equation)
As you can plainly see, there is a mass disparity between the left and right hand sides of the equations. Since we know that mass and energy need to balance between left and right sides, we know that the missing mass was released as energy. They type of energy released is unimportant right now. It is enough to know that while weíve lost mass in the system, weíve gained energy. But how much energy have we gained from this particular event? Well luckily for us, Mr. Einsteinís equation still holds true. We can use our binding energy equation to calculate the energy released from this fission event.


Now 172 MeV may not seem like a lot of energy, but when you understand that trillions and trillions of fissions take place each second in a nuclear reactor, you begin to appreciate the difference a few trillion neutrons can make. Thereís one last topic to cover in this module and it concerns why Uranium fissions in the first place.
Up Next: Lesson 1-6: Binding Energy Per Nucleon