1.6 Binding Energy Per Nucleon

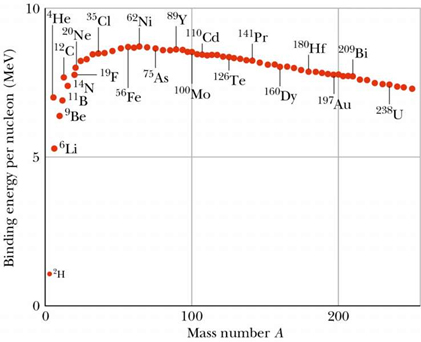
Take a look at this graph. Itís a graph of the binding energy per nucleon for the atoms on the periodic table. What is binding energy per nucleon? Well itís just as the name suggest. Its value is found by dividing the binding energy of an atom by the number of nucleons in the nucleus, but what does the graph mean?
Think of it this way. Imagine youíre holding ten tennis balls in both your hands. Your friend comes along and decides to try to pull one of the tennis balls free from your hands. To his surprise, however, youíre very good at keeping the tennis balls where they are, but the effort is overwhelming and youíre barely hanging on to all ten balls. Your friend sees that youíre struggling and decides that heís going to make you drop all those tennis balls, no matter what. He knows he canít do it by brute strength because he has already tried so he does the next best thing. He gives you one other tennis ball and tells you that now you have to hold on to eleven balls instead of ten. The next time he tries to pull a ball free, the effort is too much for your and the balls go scattering all over the floor.
This is why Uranium is susceptible to fission. Weíve already said that the nuclear force is a strong force, but that it only acts over short distances. Therefore, the further away the nucleons get from the center of the nucleus, the weaker the nuclear force gets. As you can tell by the graph above, the nuclear force reaches its maximum at about 62 nucleons. After that, the nuclear force stays constant, but the number of nucleons increases so the binding energy per nucleon decreases and you drop your tennis balls. This is why the heavier elements are less stable and this is why Uranium makes a good nuclear fuel.
Up Next: Lesson 1-7: Looking Ahead to Next Week