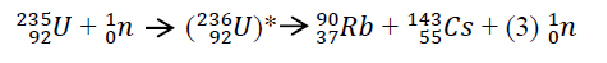
1.4 Calculating the Binding Energy
The next series of equations use the standardized model but in its generic form. Please don’t get wound around the axle because this is a generic equation. There’s nothing to be afraid of. All of you already understand the material. We’ll start with a generic atom whose chemical symbol is X
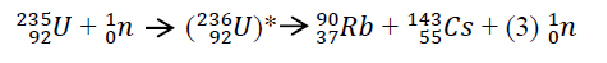
When taken at face value the equations above only make sense. We first must find the weight of the components that make up the nucleus. To do this, we multiply the number of protons (Z) by the weight of a proton in AMU. Next, we must find the weight of all the neutrons in the nucleus. As we recall from Module 1, the number of neutrons is equal to the total number of nucleons (A) minus the number of protons (Z). Once we total up the weight of the components of the nucleus, finding the mass defect is simply a matter of subtracting what the atom weighs in reality from what the atom ought to way based on the makeup of its nucleus. Once the mass defect has been found, calculating the binding energy is a breeze. We simply multiply the mass defect by our given constant.
Up Next: Lesson 1-5: Introducing the Fission Process