1.3 Size Does Matter
When we’re referring to the nucleus of an atom, size does matter. This is because the electrostatic force we mentioned earlier is a cumulative force. That is to say that the more positive charges there are within a nucleus, the more that nucleus wants to break apart. Therefore, the nuclear force within that nucleus has to be that much greater in order to hold it together. As we’ve seen with Einstein’s equation, a greater force will require a greater mass and therefore the mass defect will be larger, but suppose we were to take a single large atom and split it into two smaller atoms. What would happen then?
Before we tackle that question, we first need to be able to work with the Einstein equation.
Let’s take a look at the example we started with, a single ![]() atom. From our calculations on the first page of the module text, we know that the mass of the components of a atom is 236.9578 AMU.
From the answer I gave you, we know that the actual mass of a
atom. From our calculations on the first page of the module text, we know that the mass of the components of a atom is 236.9578 AMU.
From the answer I gave you, we know that the actual mass of a ![]() atom is 235.044 AMU. I should mention here that you will have to push the “I Believe” button. While I certainly could go through the
conversion and derivation of the constant I am going to give you, I’ve no doubt that your eyes would glaze over and you would start
shaking uncontrollably. Conversions are necessary, wonderful things, but they’re beyond the scope of this course. Suffice to say,
for the purposes of this course, 1 Atomic Mass Unit of matter is equivalent to 931.5 Mega-electron Volts. For those of you who are
wondering, a Mega-electron Volt (MeV) is a unit of energy, like a joule or a calorie. So let’s see how much energy it takes to bind a
atom is 235.044 AMU. I should mention here that you will have to push the “I Believe” button. While I certainly could go through the
conversion and derivation of the constant I am going to give you, I’ve no doubt that your eyes would glaze over and you would start
shaking uncontrollably. Conversions are necessary, wonderful things, but they’re beyond the scope of this course. Suffice to say,
for the purposes of this course, 1 Atomic Mass Unit of matter is equivalent to 931.5 Mega-electron Volts. For those of you who are
wondering, a Mega-electron Volt (MeV) is a unit of energy, like a joule or a calorie. So let’s see how much energy it takes to bind a
![]() atom together.
atom together.
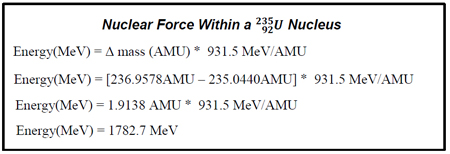
The above calculation shows that the force required to hold a ![]() nucleus together is 1782.7 MeV. When that nucleus was formed, the protons and neutrons within it gave up enough mass each to provide the nuclear binding force.
Pretty nice of them, don’t you think? Well this is a great specific example, but as we will see further along, we’re going to need to
crunch these numbers over and over. We’re going to need a generic formula and a means to calculate this force which works for all
isotopes of any element. We’re going to need a process.
nucleus together is 1782.7 MeV. When that nucleus was formed, the protons and neutrons within it gave up enough mass each to provide the nuclear binding force.
Pretty nice of them, don’t you think? Well this is a great specific example, but as we will see further along, we’re going to need to
crunch these numbers over and over. We’re going to need a generic formula and a means to calculate this force which works for all
isotopes of any element. We’re going to need a process.
Up Next: 1.4 Calculating the Binding Energy