1.2 Forces Within the Nucleus
Before we can go about solving the mystery of the missing matter, we first have to examine the forces within the nucleus. There must be something holding the nucleus together, otherwise the universe would just be a series of protons and neutrons floating in space. It certainly would be much more random, but not nearly as useful.
The Gravitational Force
This is the force that we know and understand the best. Itís the force that made the apple fall from the tree and knock poor old Issac Newton on the head. We understand gravity. Itís all around us and itís the force that keeps us from flying out into space. Gravity is an attractive force between two objects which have mass. I have mass. You have mass. The Earth has mass, and protons and neutrons have mass. Therefore, there is a gravitational force between all nucleons. They attract each other. The equation for the gravitational attractive force between objects is:
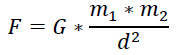
where G is the gravitational constant, m1 and m2 are the mass of the objects and d is the distance between them. Obviously the distance between nucleons is incredibly small and under normal circumstances, the size of this denominator would result in a large value for the gravitational force. However, in this case the numerator is equally small. As previously discussed the masses of protons and neutrons are so small that we had to invent units of measure for them. Because of their lack of mass the resulting gravitational attractive force is also very small. The illustration on the right represents the attractive force between nucleons. For the remainder of this module, green arrows will represent attractive forces while red arrows will represent repulsive forces.
The Electrostatic Force
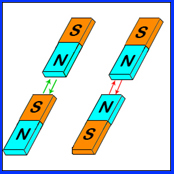
This is another force that we all intrinsically understand. Itís the force you feel when you place two magnets next to each other. Magnets have two separate poles, a positive pole and a negative pole. If you place the two opposite poles close to each other, you feel the magnets try and come together. If you attempt to push two similar poles together, the magnets repel. The figure on the right represents what you feel.
The formula for the electrostatic force is very similar to the formula for the force of gravity.
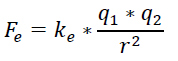
The Nuclear Force
There is another force present within the nucleus of an atom and that force is known as the nuclear force. The nuclear force is a strong force that only acts over very small distances between all nucleons within an atom. Think of the nuclear force as gravity on eleven. Itís equally strong between protons and protons, neutrons and protons and neutrons and neutrons. The nuclear force is what holds everything in the universe together.
Of course holding the universe together comes at a cost. Itís hard work keeping the universe from spinning out of control and the nuclear force comes at a price.
Everyone has heard of Albert Einstein. He was an incredibly smart guy, and if you ask anyone about Einstein
(except for particle physicists of course) they will invariably answer, E=mc
While almost everyone in the world can quote the equation, a great many fewer know exactly what it means. Count yourself into the club because weíre going to discuss it. The literal translation of the equation is this. Energy is equal to the mass of matter times the speed of light squared. So what does it really mean for us? Quite simply it means that the nuclear force takes a bit of mass away from the protons and the neutrons within a nucleus and uses that mass to create the nuclear force to hold the nucleons together. The difference between the mass of the individual nucleons and the nucleons within the nucleus is known as the mass defect, and the energy required to keep the nucleus together is referred to as the binding energy of the atom.
Up Next: Lesson 1-3: Size Does Matter