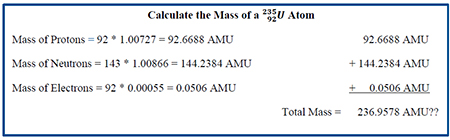
1.1 A Simple Math Problem
Let's start this chapter off with a simple math problem. We are going to total up all the masses of the particles we discussed previously to determine the mass of a atom. To do this we're going to need some information. We're going to need to know the number of protons in the atom, the number of neutrons and, of course, the number of electrons. Determining the number of protons is easy. From the previous module, we know that the lower left hand number in the standard notation is the number of protons. As you recall determining the number of neutrons is then a matter of subtracting the number of protons from the upper left hand number which is the total number of nucleons. Doing this gives of values of 92 protons, 143 neutrons and 92 electrons. We’re then going to need the atomic masses of each particle: 1.00727 AMU for a proton, 1.00866 AMU for a neutron and 0.00055 AMU for an electron. And just because I’m a nice guy, I’m also going to give you the answer. The mass of a atom is 235.044 AMU. The math in the box below shows the calculation
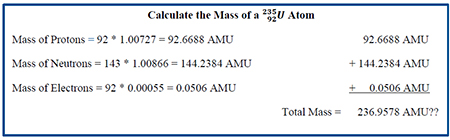
Wait a minute! How can this be? I just told you that the mass of a atom was 235.044 AMU. It's not like a used Wikipedia as a source either. I got the information from an honest to goodness physics website. Where on earth did we go wrong?
Up Next: Lesson 1-2: Forces Within the Nucleus