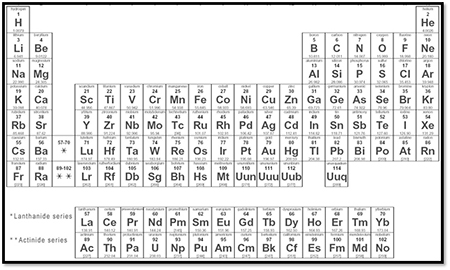
1.4 The Periodic Table of Elements

Behold everything in the Universe. Thatís right. I said everything. Everything in the universe is made up of atoms whose information is contained in this graphic. Impressive, donít you think? Actually, the Periodic Table of Elements gives us quite a bit of information required for this course. There are various permutations of this table available on line for viewing, but for our purposes, simple is best. Simple you say? It looks pretty complicated to me. Letís look at the information for a single element in order to see all the things that the Periodic Table has to offer.
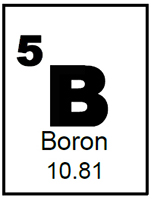
Letís look at the Periodic Table information for the element Boron. The first thing that stands out is the big ĎBí in the middle. That ĎBí is Boronís chemical symbol. Each element in the periodic table has its own unique symbol. Some of them, such as Boron, have a symbol that makes sense. Others, such as Lead, whose chemical symbol is Pb, make less sense, but whether they make sense or not, each element has its own symbol.
The next thing to notice is that the table not only gives us the symbol, but it gives us the name of each element. This is quite helpful just in case we didnít know that Hg was the symbol for Mercury.
The next two pieces of information are the most pertinent to our discussions. The first is the atomic number of the element, that is to say the number 5 in the upper left hand corner. Each element has, not only a unique symbol, but a unique atomic number. The atomic number for each element is based on the number of protons within the nucleus. Putting it a different way, if a Boron atom were to have 6 protons, it would no longer be Boron, but instead would be carbon. A Boron atom will always have 5 protons just as an atom of Uranium will always have 92 protons.
So Boron has 5 protons, but we said that the nucleus contains both protons and neutrons. How come the geniuses who created this table didnít put the number of neutrons in that little square? Well it turns out they did it for a reason, as geniuses often do and weíll discuss that reason later in this module, but it turns out that we donít need to list the number of neutrons in an element. We can calculate the number using the remaining information on the periodic table.
The number which we havenít discussed yet is the atomic mass of the element. In this case, Boron has an atomic mass of 10.81 Atomic Mass Units. Since we know that protons and neutrons each have an approximate atomic mass of 1Atomic Mass Unit and we know the number of protons in a Boron atom from its atomic number, we can calculate the number of neutrons in an atom by subtracting the number of protons from the rounded atomic mass of the element. Put another way, since we declared electrons as non-contributors to the mass of the atom, the number of nucleons in an atom is approximately equal to the atomic mass off the atom.
Up Next: Lesson 1-5: Setting the Standard