1.5 Setting the Standard
Scientists are, in general, a lazy bunch, especially when it comes to writing. They’ll spend all day pouring over equations, but when it comes to words, the shorter the better. As you can imagine, this presents a serious problem when they’re writing about atoms. Scientists would have staged a revolt if they had to write, “A Helium atom with 2 protons and 2 neutrons and a Lithium atom with 3 protons and 4 neutrons results from the decay of a Boron atom with 5 protons and 6 neutrons.” Come to think of it, their readers would have staged a revolt as well.
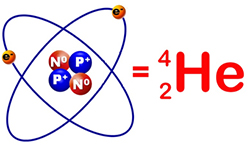
Because of this, and because we’re going to be discussing many other atoms during this course, a shorthand has been developed. That shorthand is called, “Standard Notation.” Take a look at the figure on the right. On one side of the equation we have an actual Helium atom, and on the other side of the equation, we have the standard notation representation of a Helium atom. The first thing we notice when we look at the standard notation is that the chemical symbol of the atom the chemical symbol is always written on the right. The two numbers on the left, though, are the items that we are going to be concerned about. The number on the lower left, in this case the number 2, represents the atomic number of the element, that is to say, the number of protons. The number on the upper left represents the total number of nucleons contained within the atom. As we said previously, the total number of nucleons is equal to the number of protons added to the number of neutrons. Since a Helium atom has 2 protons and 2 neutrons, the upper left hand number is 4.
Now that we understand standard notation, we can rewrite the long statement in the first paragraph as
![]() This is much simpler and much quicker which is why
scientists use it. I did want to point on one term from the previous equation, and that is the term. This is what we use to
represent a neutron, and as we examine it, it only makes sense. A neutron is a single nucleon, but it is not a proton hence the
lower left hand number is a zero.
This is much simpler and much quicker which is why
scientists use it. I did want to point on one term from the previous equation, and that is the term. This is what we use to
represent a neutron, and as we examine it, it only makes sense. A neutron is a single nucleon, but it is not a proton hence the
lower left hand number is a zero.
Up Next: Lesson 1-6: Isotopes, the Atomic Fraternal Twin