1.1 What is an atom?
Atoms are everywhere. Atoms are everything. They’re in the air you breathe, the food you eat. Everything in the universe is made up of atoms. They’re pretty important.
The concept of the atom was first conceived in ancient Greece. Greek philosophers Leucippus and Democritus first developed the concept of the atom. They theorized that there were certain small particles which could not be divided any further. They named these particles atoms, from the Greek word atomos meaning indivisible.
Of course we know now that atoms can indeed be split, but it’s interesting to note that the classical definition of an atom has stood the test of time. Simply put, an atom is defined as the smallest component of an element that retains the chemical properties of that element.
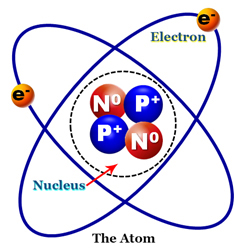
As previously discussed, we now know that atoms are made up of even smaller, common sub-components, and that these sub-components are interchangeable. Each atom essentially has two separate regions. There is the nucleus, which will be our primary focus for this course and the electrons which are orbiting the nucleus. In many ways, an atom is similar to our solar system where the nucleus is the sun and the electrons are the individual planets.
There are many real and theoretical components of an atom’s nucleus, but for the purposes of this course, we are only going to concern ourselves with the two major components, namely protons and neutrons. It is important to remember that any sub-atomic particle found in the nucleus be it proton, neutron or anti-neutrino are referred to as nucleons.
Up Next: Lesson 1-2: Property Values